electrons, protons, and neutrons. The number and arrangement of these

protons, neutrons, and electrons make up all the planets, stars, gas,

protons/electrons: 2. # neutrons: 2. Atomic mass: 4.002602

which in turn are made of protons, neutrons, and electrons, like so.

All atoms have different numbers of protons, neutrons and electrons.
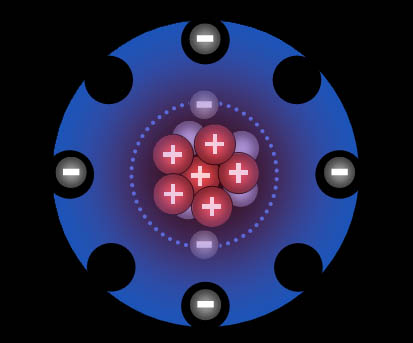
It has six protons, six neutrons, and six electrons.

Model of a carbon atom showing the electrons, protons, and neutrons.

(Electrons dance all around the protons and neutrons like crazy little

protons and neutrons. These electrons move at lightspeed

protons, neutrons, and electrons. Protons and neutrons
Organization of atoms 1) Nucleus - contains protons and neutrons,

Learn how atoms are formed from electrons, protons and neutrons and how the

protons and neutrons can undergo fusion to form heavier atomic nuclei.
are three subatomic particles of protons, electrons, and neutrons (quark

electrons than protons. An ion is not neutral an so it is radioactif.

The oxygen atom has 8 protons, 8 neutrons, and 8 electrons

their protons, neutrons, electrons

Protons Neutrons Electrons Worksheet. Mass, atomic changes pdf what happens

The Protons, electrons and neutrons are known as subatomic or elementary

charge, electrons, protons, neutrons, and matter




No comments:
Post a Comment